Understanding and Differentiating Esters
Trenbolone Acetate, Trenbolone Enanthate, Testosterone Cypionate, Testosterone Propionate...
But what are these names that follow molecules? Does this have an influence on the way to take my products?
The answer is YES: the name of theester is very important for the construction of your cure.
One of the most misunderstood topics in the steroid world is theester : i.e. the mechanism by which steroids such as testosterone cypionate, testosterone enanthate, and esterified injectable Sustanon or Trenbolone enanthate or acetate work in your body.
If you take a look on the internet, you will undoubtedly find countless articles that consider one form of a steroid to be much more effective than another.
Arguments about the superiority of cypionate over Enanthate or even Sustanon over all other testosterones are, of course, very common but are misconceptions.
Indeed, each ester has its particularity and, used intelligently in your treatment, it will be your perfect ally to obtain the results you desire.
What is an Ester?
Take the example of testosterone which comes in different esters : cypionate, enanthate, propionate, heptylate, caproate, phenylpropionate, isocaproate, decanoate, acetate… the list goes on and on.
In all these examples the “mother” hormone is testosterone, which has been modified by adding an ester to its structure.
The following question then arises: What is the difference between the different “esterified” versions of testosterone in terms of their use?
It is simply a question of the speed of assimilation of the molecule by the organism.
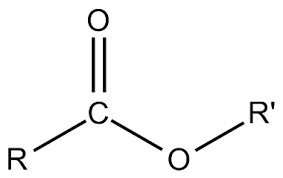
This chain is usually attached to the steroid hormone at the 17 carbon position (beta orientation).
The esterification of a anabolic steroid / injectable androgen essentially does one thing, it slows down the release of the active substance of the molecule from the injection site.
This happens because theester will notably have lowered solubility in water or oil containing the steroid.
This will have the effect of forming a deposit in the muscle tissue, from which circulation will pass slowly and the steroid will be taken up in small amounts by the blood.
Generally, the more soluble the ester chain, the faster the hormone will be released.
The slowing down of hormone release is a great advantage in a cure. This is because without an added ester, free testosterone (or other steroid hormones) would only remain active in the body for a very short period of time (usually a few hours). This would therefore require a cumbersome and unpleasant daily injection schedule if one wants to maintain a high and stable level of testosterone in the body.
With the addition of an ester, the athlete can therefore reduce the frequency of injections.
Adding an ester thus amounts to providing users with ease of use by making the treatment much less restrictive.
Is one ester stronger than another?
A bit of technique now.
We have therefore seen that esterification temporarily deactivates the steroid molecule and thus allows assimilation by the body that is better distributed over time.
With a blocking chain in the 17 beta position, binding to the androgen receptor is not possible, so it cannot exert any activity in the body.
This will restore the hydroxyl (OH) group to the 17 beta position, which will cause the steroid to bind to the appropriate receptor.
It is therefore after the disappearance of the ester that the steroid is able to have an effect on skeletal muscle tissue.
You are already beginning to understand why considering Cypionate to be stronger than enanthate is nonsense. Indeed, your muscles only distinguish free testosterone, regardless of the ester that was used for the administration of the molecule.
There are many different esters that are used with anabolic steroids but again, they all do the same thing: reduce solubility.
A ester as Propionate for example will slow the release of a steroid for a few days, while the slowdown will be several weeks with a decanoate ester.
Esters therefore have no effect on the estrogen or DHT (dihydrotestosterone) aromatization tendency of a steroid or on the overall potency of the compound.
The differences in results and side effects that can be noticed by bodybuilders who have used different esterified versions of the same basic steroid are just matters of timing.
For example, Enanthate will cause estrogen related issues more easily than Sustanon, quite simply because Testosterone Enanthate levels will have their peaks and troughs much sooner (1 to 2 weeks for enanthate versus 3 to 4 weeks for Sustanon).
Given an equal testosterone blood level, there would be no difference in aromatization or DHT conversion rate between the different esters. It's just the speed of this mechanism that differs.
The different ester profiles in detail.
Now that we know what an ester is and how it works, we'll break down the most popular ones in alphabetical order. This will help you to better control and optimize your cures and thus obtain better results.
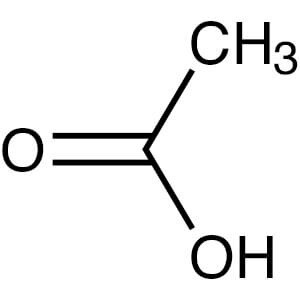
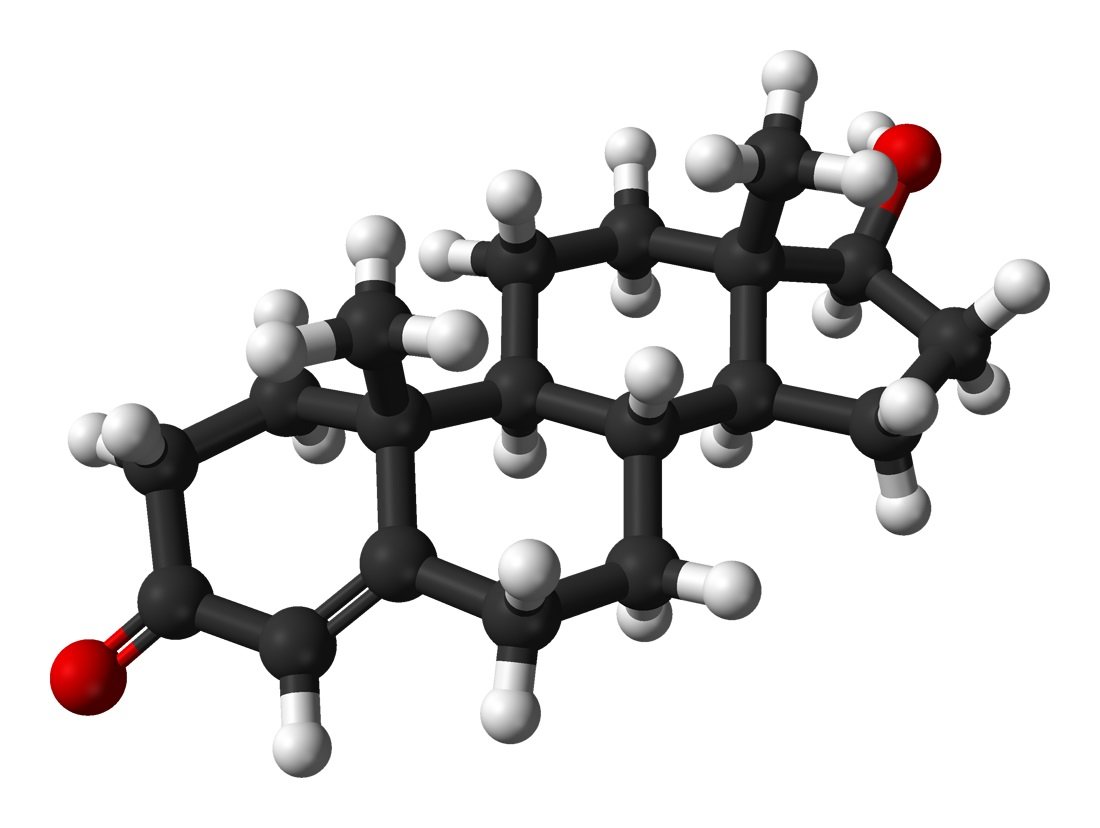
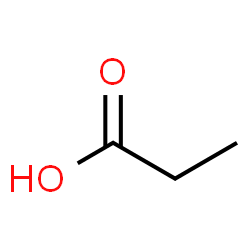
Acetate
C2H4O2 chemical structure.
Also known as acetic acid. Acetate Ester delays the release of a steroid for only a few days. Contrary to what you may have read, acetate esters do not increase the tendency for fat burning. Again, there is no known mechanism that would allow acetate to burn fat.
This ester is used on Primobolan (metenolone acetate) oral tablets, on Finaplix (trenbolone acetate - “Tren A”) and occasionally for testosterone.
Cypionate
Chemical structure C8H14O2.
Cypionate is a very popular ester in the United States. Its release duration is almost identical to enanthate (10-14 days). In the USA, athletes generally think that cypionate is more potent than enanthate. Yet there is very little difference between the two. The Enanthate ester is actually slightly smaller than cypionate, and so it releases a tiny (maybe a few milligrams) amount of steroid more when compared.
Decanoate
Chemical structure C10H20O2.
Also known as decanoic acid. The decanoate ester is most often used with nandrolone (as in Deca-Durabolin). It is present in virtually every corner of the world. Testosterone Decanoate is also the longest acting active constituent in Sustanon, greatly extending its release duration. Release time with decanoate compounds is just under a month. However, it has recently been found that the levels seem to drop significantly after two weeks. To keep blood levels more even, athletes will follow a weekly injection schedule.
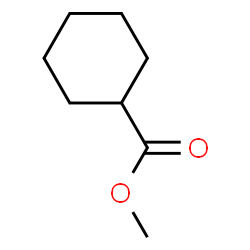
 Enanthate
Enanthate
Chemical structure C7H14O2.
It is one of the most important esters used in steroid manufacturing, most commonly seen with testosterone. It is also used in other compounds such as Primobolan Depot.
Enanthate will maintain a balanced level of the hormone for about 10 to 14 days.
Although enanthate compounds are often injected as part of medicine on a monthly or even bi-weekly basis, athletes, on the other hand, will prefer weekly or bi-weekly injections to help maintain a consistent testosterone level.
Isocarpoate
Chemical structure C6H12O2.
This esters has a slightly shorter lifespan than enanthate and is only used with testosterone in “blends” of the Sustanon or Omnadren type.
Phenylpropionate
Chemical structure C9H10O2.
The phenylpropionate ester will prolong the release of the active steroid for a few days longer than the propionate ester.
To maintain propionate-like blood levels, injections are given at least twice a week. Deca-Durabolin is the most commonly seen product with a phenylpropionate ester (nandrolone phenylpropionate - "npp") but it is also used with testosterone in Sustanon and Omnadren.
Propionate
C3H6O2 chemical structure.
Also called Carboxyethane or propionic acid. Propionate ester will slow the release of a steroid for several days.
To allow blood levels to rise rapidly, the propionate compounds are usually injected two to three times per week. One of the most well-known steroids is testosterone propionate.
Undecanoate
Chemical structure C11H22O2.
Undecanoate is not a very common ester. It only appears to be used in the preparation of nandrolone (Dynabolan) and oral testosterone undecanoate (Andriol). Since this ester is chemically very similar to undecylenate (There are only two more hydrogen atoms), it has a similar release time (About 2-3 weeks).
There is no reason to believe that it has any unique properties compared to other esters because it is used in the oral preparation Andriol.
Andriol actually works very poorly at delivering testosterone, reinforcing the idea that oral administration really isn't the ideal use for esterified testosterone.
Undecylenate
Chemical structure C11H20O2.
This ester is very similar to decanoate, containing just one more carbon atom. Its release time is also very similar (around 2-3 weeks), possibly extending a day or two longer than decanoate.
Undecylenate seems to be exclusively reserved for the veterinary preparation Equipoise (boldenone Undecylenate), Equipoise works very well for athletes.
Again, weekly injections are the most common.
Conclusion
Each ester therefore has its particularity concerning the release of the hormone in the body. But this release time is also dependent on another factor, a technical factor: the weight of theester.
The more the chainester will be longer the heavier it will be. In the case of testosterone enanthate for example, 250mg of esterified steroid (Testosterone enanthate) is equal to only 180mg of free testosterone for 70mg of enanthate.
You will understand, propionate being the shortest ester, it is therefore this esters which is the lightest.
So although the advent of esters is a valuable advance in the steroid field, they do not alter the anabolic potency of the steroid in any way.
Testosterone remains testosterone, trenbolone rest of the trenbolone, etc…. Some will claim that a form ofester of this or that hormone is much better than another: it is a totally unfounded and erroneous view.
You now have all the cards in hand to best compose your cures, and if in doubt, do not hesitate to contact one of our advisers who will be happy to guide you.
Good cure.
The Top Steroids Online team.
Share this post
Laisser un commentaire
You must be logged in to post a comment.





 Enanthate
Enanthate








Comments (7)
O undecanoate of testosterone é fórmula do restorative hormonal do NEBIDO. Gostaria de saber se o decanoato de testosterona teria o mesmo efeito?
Buenos dias,
Tendrá los mismos efectos
acetic acid não ester, carboxylic acid
Thank you for this in-depth explanation on esters, we will go to bed less stupid !!!!
Salve, volevo chiedere turns out a programma da seguire rigardante prodotti e modalità di assunzione dei prodotti che dovrei acquistare da Voi.
Grazie
Buongiorno,
Ti invito ad avvicinarti ai nostri coach che sono felici di darti cure su misura e di usufruire dei loro preziosi consigli.
Puoi contattarli tramite questo link: https://it.top-steroids-online.is/conseils-cure-gratuits/
Excellent article on esters.